by Nathan Favini, MD, MS, Forward Medical Lead, and Richard B. Lanman, MD, Forward Advisor
Recently, we discussed the differences between PCR and antibody tests for the coronavirus that causes COVID-19 and how we use each test in the care of our members here at Forward. We didn’t talk much about the FDA’s role and how we think the FDA could lead the nation toward a better approach to COVID-19 testing. In this post, we’ll discuss our approach to how rapid antibody tests should be used and how the FDA might improve its stance to help healthcare providers rapidly scale testing to more people.
As a reminder, antibody tests look for proteins that are produced by your body in response to the SARS-CoV-2 virus. The test is performed on your blood, often obtained by a simple finger-prick or blood draw. Rapid antibody testscan provide results in just 10–15 minutes and cost roughly one fifth the price of a PCR test. However, different rapid antibody tests have sensitivities and specificities that vary across a 75–90% range. This means that some tests may produce a significant number of false positives and negatives. There are hundreds of manufacturers producing COVID-19 antibody tests, and each manufacturer’s kit has different test characteristics. The FDA has taken different attempts at regulating these tests, and we have some ideas for an approach that we believe would most effectively help us on the front line of the pandemic. But first, let’s provide context on the FDA’s approaches.
Until last week, the FDA had allowed state regulation of COVID-19 antibody tests and not required antibody test providers to receive a FDA emergency use authorization — essentially, a manufacturer could sell its test if it had done its own validation and gave notice to the FDA. This led to the market being flooded with tests of questionable reliability. As a result, we and others have had to do our own validation studies of the testing kits. Thankfully, the FDA now requires manufacturers to submit validation data, and it appears they may remove unreliable tests from the market. We welcome this degree of oversight, though, we are concerned that the FDA may remove tests unnecessarily. A test should not have to be perfect to be on the market. The thresholds of 90% sensitivity and 95% specificity that FDA is considering are too high and will disqualify most tests that are available today. In our view, as long as a test’s performance characteristics are well understood and transparent, their results can still be useful when interpreted by a physician in the context of patient care, as we’ll show in our hypothetical testing algorithm below.
A separate angle of the FDA’s regulation of antibody tests is on who can perform them. To date, very few rapid antibody tests have been authorized for use outside of high-complexity CLIA-certified labs. As you can imagine, the US doesn’t have enough high-complexity labs to quickly test our hundreds of millions of residents. We don’t know why the FDA has restricted many of these tests exclusively to high-complexity labs. There’s nothing high-complexity about running these tests — you put a drop of blood on the test, and it runs itself. From this perspective, they are no more complicated than rapid HIV or urine pregnancy tests. Admittedly, these tests don’t have remotely the degree of validation and use-testing performed to receive the traditional FDA approval of those HIV or urine pregnancy tests, but that’s the idea behind the FDA’s emergency use authorizations (EUAs) in the first place. One theory is that the FDA may simply be trying to limit potential downside exposure of bad tests, by limiting the number of tests that can be run. While we understand the concern here, we believe the FDA has the ability to lead, safely, and to massively expand the testing throughput of the US. They can do so by starting with what they’re doing now (requiring an EUA), and allowing easily-administered tests to be performed at the point-of-care so long as they are used as part of a testing algorithm like the one we propose below.
Acknowledging the limitations of rapid antibody tests and placing them in an appropriate testing algorithm would allow them to be used responsibly and to maximize the number of people who can be tested today. By avoiding rapid antibody tests in settings where they are not accurate (e.g. the first 7 days of symptoms) and confirming the results in settings where we know the tests may be incorrect, we can rely on them in the situations where they are most useful. In the figure below, we propose a rational testing algorithm for COVID-19 that relies on rapid antibody testing, PCR testing and confirmatory ELISA testing. This approach would allow us to use resources efficiently and make testing available to more people.
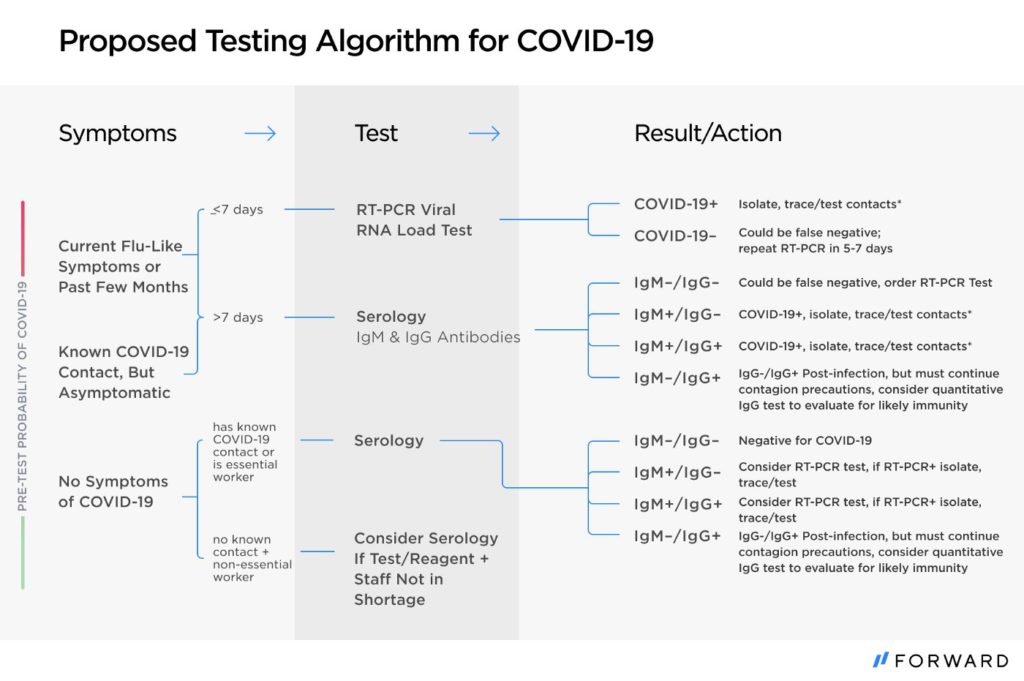
For instance, when someone has been symptomatic for more than seven days, a provider can use a serology test as the first line of testing. If it is negative, a PCR test should be performed to confirm the result given the likelihood of a false negative. If the same person were to test positive on both the IgM and IgG tests, confirmatory PCR testing would not be necessary, since their symptoms and both antibody tests turning positive make this very likely to be a true positive result.
With a clear understanding of their limitations, and a strategy for when to use them, rapid antibody tests are a powerful asset to getting our country the testing supply that is needed. We urge the FDA to provide transparent evaluations of rapid antibody tests and keep any test that is above 75% sensitive and specific on the market, as long as it is used in an appropriate testing algorithm. We urge the FDA to evaluate the need to limit easy-to-perform tests to only high-complexity CLIA-certified labs. Now is the time the FDA can capitalize on its position and expertise, and greatly increase our ability to understand and fight the pandemic.
Dr. Nate Favini is Medical Lead at Forward, the preventive primary care practice combining top-rated doctors and advanced medical technology. Dr. Richard B. Lanman, a Medical Advisor to Forward, has an extensive career at the intersection of health and innovation, most recently serving as Chief Medical Officer of Guardant Health.
Forward stays on top of the latest research and current best practices for COVID-19 prevention, treatment, testing, and vaccinations. Our physician-led COVID-19 care program is a one-stop-shop for symptom assessments, guidance, and gold-standard care.